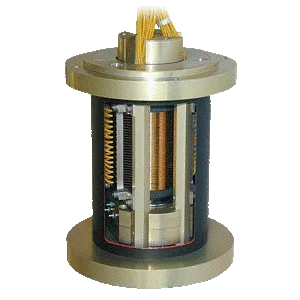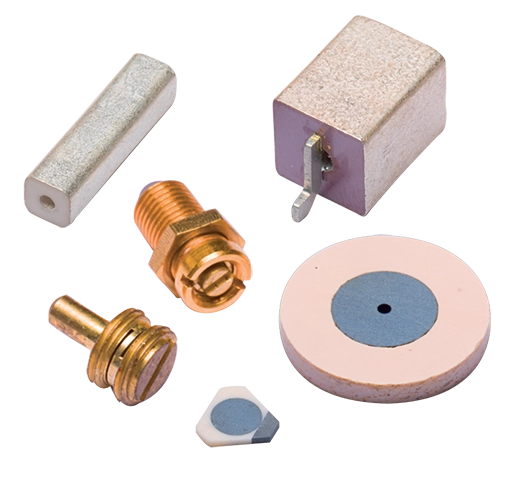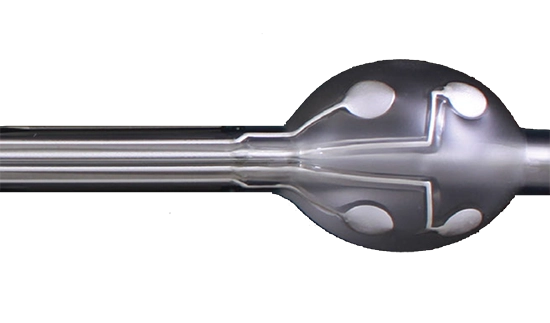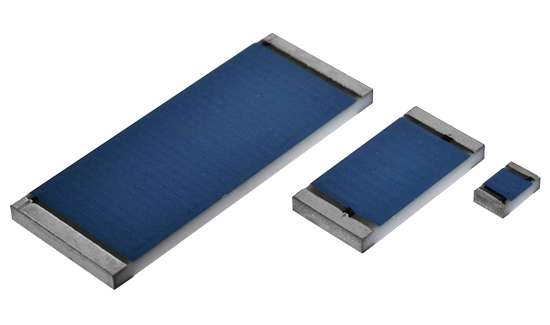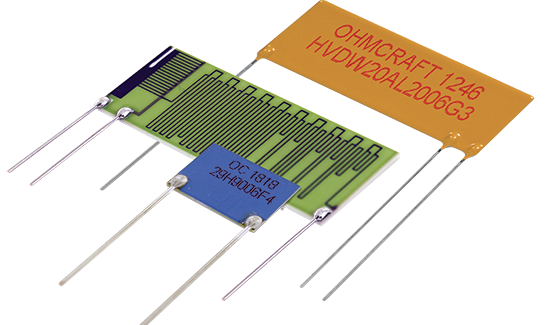
Ohmcraft Resistors
Choose or Design Your Product
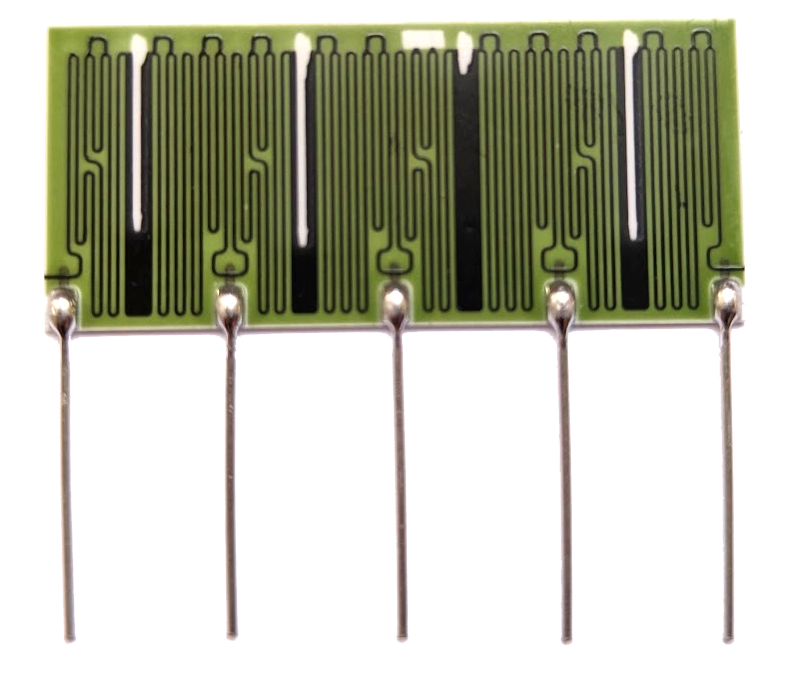
Micropenning, a “direct write” technology, is Exxelia Ohmcraft’s proprietary technique for manufacturing its family of high voltage resistors.
Exxelia Ohmcraft manufactures catalog and custom precision surface mount resistors and leaded through-hole resistors tailored to meet specific application requirements, ensuring utmost reliability.
All of our high voltage resistors and dividers can be screened and qualified under rigorous standards such as MIL-PRF-55342, MIL-PRF-49462, and NASA EEE-INST-002 (Level 1 & 2).
25 ppm/°C
TCR down to
0,1%
high precision
tolerances
Up to 100
kV
Highlighted products







Frequently Asked Questions
A full-service partner who can collaborate on product design, rapid prototyping, manufacturing, testing, and supply chain services is ideal. Consolidating all activities under one roof can reduce costs and time to market. It also allows for problems to be addressed quickly and eliminates the finger-pointing when multiple suppliers are involved. You should choose a partner who will complement your own team, adding value end-to-end.
At Micropen we have expertise from design through manufacturing and responsive support throughout the product lifecycle. Our flexible printing manufacturing services can meet the needs of any company, from a start-up to a major OEM.
It is important to choose a partner who can meet your production requirements now and into the future. The manufacturer should be agile and flexible in regard to your demand changes and able to meet your deliveries as agreed. This requires that they have a well-understood supply chain of raw materials and lead times. Make sure to ask about this when selecting a partner. At Exxelia Micropen we have the resources and experience to respond to growth and demand increases.
This is especially important in today’s roller-coaster supply chain economy. We have excellent partnerships with suppliers of ink, substrates, and other raw materials and have a well-established process for supply chain management and production scheduling.
The most successful partnerships are long-term and collaborative. The resources on each team should have a shared understanding of the objectives and requirements,
and the chosen supplier should be seen as an extension of your team. This allows challenges to be solved quickly and assures that the project will be completed on
time and within budget. Regular meetings, passing files back and forth, and engaging experts in different functions of the organization are all elements of a partnership and lead to the success of the project. Your partner should be proactively communicating throughout the product lifecycle. Your partner may also consider investing in the partnership for the long-term. Over time, as you work together, you’ll be able to apply learnings and efficiency to not only one product but a portfolio of products in a cost-effective and optimized way.
At Exxelia Micropen, we are committed to working collaboratively, starting early in the product design stage. We will share with you our accomplishments, manufacturing controls, and quality assurance process steps. We want you to get to know us, and the earlier we can engage with you as an integrated partner to your design team, the better. Most of our customers have been with us for years and we value a platform approach where we can apply our learnings to product extensions and next-generation designs.
There are complex requirements in the healthcare industry, and a proven track record in the understanding of proper documentation, revision control, and maintenance of device history records and safety regulations will go a long way to ensure that your project is successful. An experienced partner can provide advice on design, suitableinks, techniques, and steps to optimize the process.
This can lead to valuable time and cost savings over the course of the project. There is no substitute for experience in partnering on the design and manufacturing of printed electronics for the implantable medical device market. Exxelia Micropen has over 25 years of experience in working with top-tier medical device companies, printing electronics on endotracheal tubes, ablation devices, balloon catheters, etc.
Our engineering and development teams have the necessary technical expertise, and our production, test, and quality teams understand what it takes to manufacture volume devices while maintaining competitive pricing. We understand that we are adding value to devices that will be used to save or enhance a patient’s life.
Still have questions ?